Frustrated European Union member states faced shortages and a slow rollout of vaccine supplies.
Leaders of countries like the Czech Republic, Hungary, and Austria are looking to eastern nations seeking Russian and Chinese vaccines which were not previously approved by the European Commission’s unifying strategy.
On Feb.16, the first batch of the Chinese vaccine Sinopharm arrived in Budapest making Hungary the first EU member state to purchase and authorize the use of Chinese vaccines.
Hungarian Prime Minister Viktor Orban was photographed receiving the Sinopharm vaccine in a recent Facebook post stating, “Today is an important day because (on) this day we are starting to vaccinate with Chinese vaccines,” said Orban.
This public endorsement came two days after Hungarian president Janos Ader also opted for the Sinopharm vaccine which boasts a 72.5 per cent efficacy rate according to the Wuhan Institute of Biological Products.
According to government-collected data, as of Feb. 28, over 677,682 Hungarians have received at least one dose of the vaccine, while over 249,499 have received two doses.
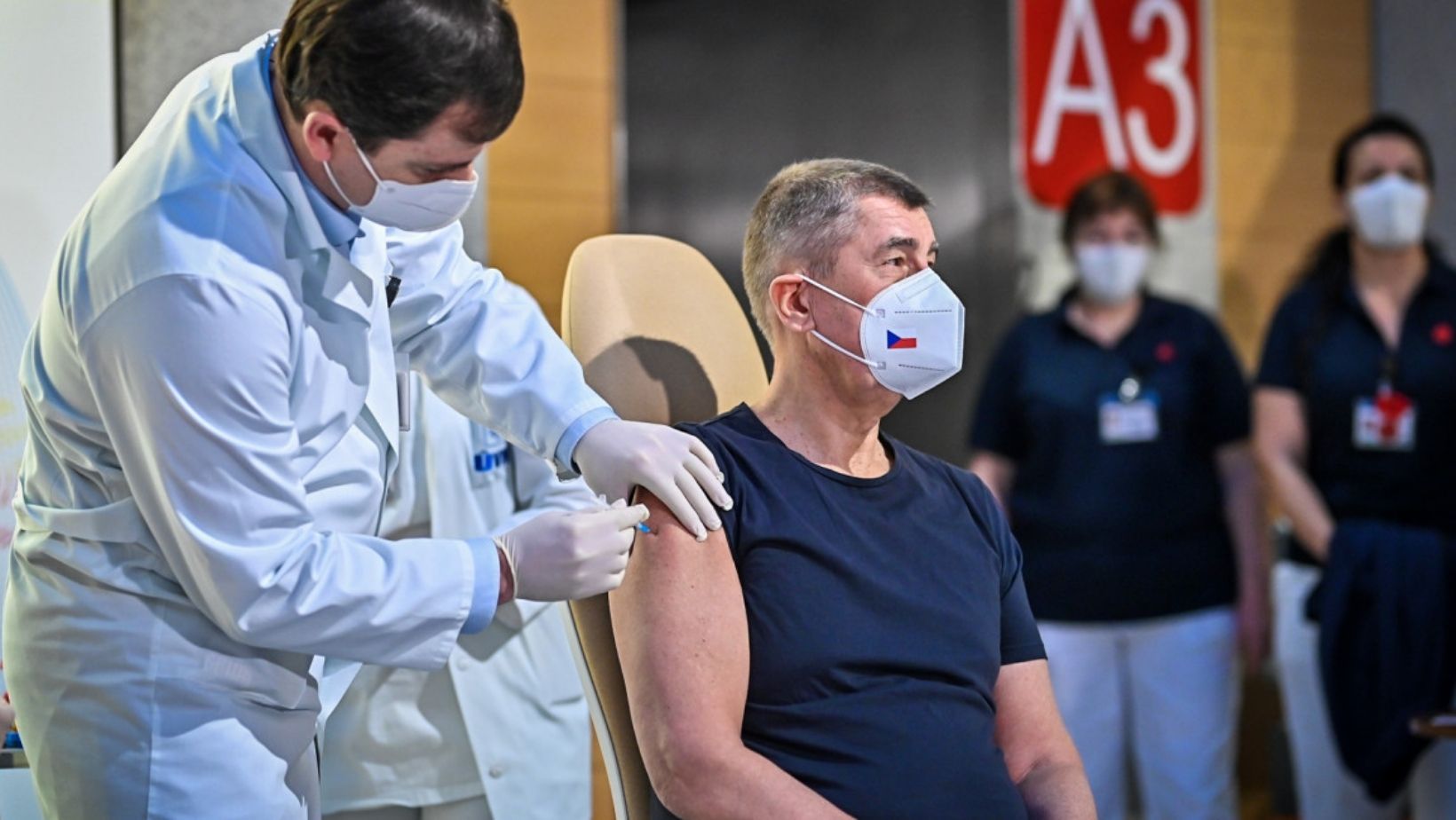
In the Czech Republic, President Miloš Zeman along with Prime Minister Andrej Babiš, reached out to Russian President Vladimir Putin requesting an order of vaccines from the Russian manufacturer Sputnik.
President Zeman deemed the Sputnik V vaccine permissible once it has been officially certified by the Czech State Drug Control Institute (SUKL).
On Monday, Slovakia became the second European country to announce it had purchased the Sputnik V vaccine, securing 2 million doses before it’s been officially approved by the country’s drug administration.
In Vienna, the Austrian government is said to be in negotiations with Moscow concerning its own procurement of the Sputnik V vaccine.
Early findings from clinical trials indicate the Sputnik V vaccine was 91.6% effective against symptomatic Covid-19 infection.
Meanwhile, the European Commission has yet to sanction the use of Sputnik V or Sinopharm and have given three conditional marketing authorizations to Pfizer, Moderna, and AstraZeneca, while several other vaccines are at different stages of assessment.
The EU has also doubled its contribution to the global initiative COVAX, which pledges to “secure fair and equitable access to safe and effective COVID-19 vaccines in low and middle-income countries,” according to the commission’s website.
The EU has now donated 1 billion Euros in grant funding to the COVAX initiative with the goal of administering 1.3 billion doses for 92 low and middle-income countries by the end of 2021.
-
NEWSLETTER
Subscribe for our daily news